Big News! SyncoZymes (Shanghai) Co., Ltd , The world's first NMN raw material passed FDA NDI certification.
After strict review by the professional committee of the US FDA (US Food and Drug Administration) authoritative organization, on May 17, 2022, SyncoZymes (Shanghai) Co., Ltd. officially received the FDA's confirmation letter (AKL): NMN raw material successfully passed NDI (New Dietary Ingredient) approval.
15
2024-01
Australian scientists study proves that NMN can strengthen bones
Recently, Australian researchers published scientific research results in the Journal of Gerontology: Series A: NMN can reduce the aging of human bone cells and promote bone healing in osteoporotic mice. "The findings demonstrate NMN as an effective and feasible therapeutic candidate to prevent osteoporosis and enhance bone healing in older adults with osteoporosis," the authors said.
19
2023-05
New research in Europe: NMN can improve the antiviral activity of CD8 T cells, a specific immune cell of patients with hepatitis B.
For patients with chronic hepatitis B, the high level exposure of hepatitis B virus-specific immune cells (CD8 T cells) to pathogens such as hepatitis B virus has caused DNA damage and dysfunction of CD8 T cells. Identifying the cellular process behind CD8 T cell damage is helpful to develop the treatment of chronic hepatitis B.. Recent studies have shown that NAD+ depletion is the basis of CD8 T cell depletion.
07
2023-01
Japanese scientists have found that supplementing NMN can treat retinal dysfunction caused by cardiovascular diseases.
As we grow older, we are more likely to suffer from cardiovascular complications-retinal dysfunction. The retina is the part of the eye responsible for receiving light signals, which can easily lead to retinal dysfunction due to cardiovascular diseases (such as carotid artery occlusion).
16
2022-12
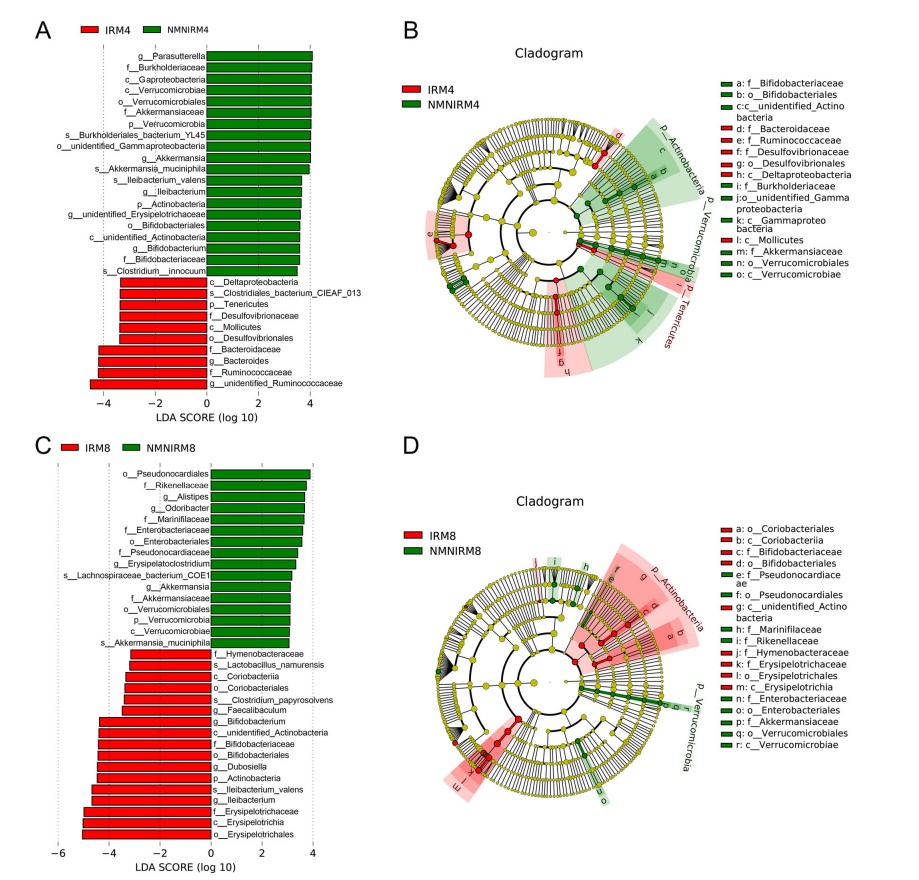
A new stage of the epidemic? Many studies have proved that NMN is helpful to prevent and treat Covid-19.
Since December 2022, due to the reduction of virus toxicity, China has started to optimize epidemic prevention and control, precise prevention and control, and scientific prevention and control, including measures such as lifting temporary control areas and not checking nucleic acid certificates when going in and out of major places, which shows that the dynamic optimization of epidemic prevention and control has entered a new stage.
12
2022-12
COVID-19 nemesis? Nature: Ursodeoxycholic acid (UDCA) can prevent Covid-19 infection by inhibiting ACE2 receptor!
Studies by most authoritative medical institutions in the world show that ursodeoxycholic acid (UDCA) has unexpected therapeutic effects on diseases that cannot be treated in medicine at present. In the late 1980s, the U.S. Food and Drug Administration (FDA) officially approved ursodeoxycholic acid as a drug for treating liver diseases. At present, the patent period of this drug has expired, and now it can be obtained as a generic drug.
Search

Tel:021-68187180
Fax:021-68187179
E-mail:services@syncozymes.com
Address: No. 1199, Landian Road, Zhoupu Town, Pudong New Area, Shanghai

Copyright ©SyncoZymes (Shanghai) Co., Ltd. 沪ICP备09012407号 By:300.cn
-
-
-
Customer service contact information
Service hours:9:00-18:00
Contact number:
I want to leave a message:
-